potassium
(K) [po-tas´e-um]Since most foods contain a good supply of potassium, potassium deficiency (hypokalemia) is unlikely to be caused by an unbalanced diet. Possible causes include cushing's syndrome (due to an adrenal gland disorder) and fanconi's syndrome (the result of a congenital kidney defect). The cause could also be an excessive dose of cortisone, prolonged vomiting or diarrhea, or thiazide diuretics, which are administered for treatment of hypertension. Signs of potassium deficiency can include weakness and lethargy, rapid pulse, nausea, diarrhea, and tingling sensations.
If the body absorbs enough potassium but the element is not distributed properly, various disorders may develop. Thus an abnormally low content of potassium in the blood may result in an intermittent temporary paralysis of the muscles, known as familial periodic paralysis.
Potassium deficiency can be treated by administration of potassium supplements. There is a large variety of these preparations. Some are liquids, some are powders to be dissolved in liquids, and some are slow-release tablets that dissolve in the intestine. All can cause gastrointestinal irritation. For many persons on diuretic therapy for hypertension, potassium deficiency can be avoided by increasing their consumption of potassium-containing foods, such as bananas, dates, prunes, and raisins, and potassium supplements are not needed. Potassium supplements are never given to patients receiving potassium-sparing diuretics such as amiloride, spironolactone, or triamterene. If the difficulty lies in the body's use of potassium, treatment is concerned with the primary cause of the deficiency.
potassium acetate
Pharmacologic class: Mineral, electrolyte
Therapeutic class: Electrolyte replacement, nutritional supplement
Pregnancy risk category C
Action
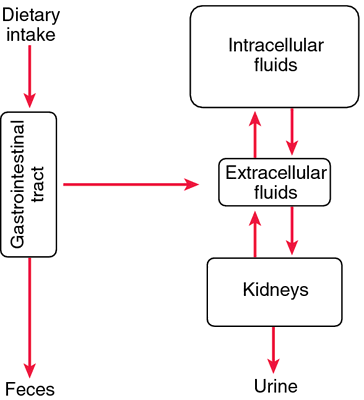
Maintains acid-base balance, isotonicity, and electrophysiologic balance throughout body tissues; crucial to nerve impulse transmission and contraction of cardiac, skeletal, and smooth muscle. Also essential for normal renal function and carbohydrate metabolism.
Availability
Concentrate for injection: 2 mEq/ml in 20-, 50-, and 100-ml vials; 4 mEq/ml in 50-ml vials
Indications and dosages
➣ To prevent or treat potassium depletion; diabetic acidosis; metabolic alkalosis; arrhythmias; periodic paralysis attacks; hyperadrenocorticism; primary aldosteronism; healing phase of burns or scalds; overmedication with adrenocorticoids, testosterone, or corticotropin
Adults: Dosage highly individualized. For potassium level above 2.5 mEq/L, give 40 mEq/L as additive to I.V. infusion at a maximum rate of 10 mEq/hour; maximum daily dosage is 200 mEq. For potassium level less than 2 mEq/L, give 80 mEq/L as additive to I.V. infusion at a maximum rate of 40 mEq/hour (with cardiac monitoring); maximum daily dosage is 400 mEq.
Children: Dosage highly individualized; up to 3 mEq/kg or 40 mEq/m2/day as additive to I.V. infusion.
Contraindications
• Acute dehydration
• Heat cramps
• Hyperkalemia
• Hyperkalemic familial periodic paralysis
• Severe renal impairment
• Severe hemolytic reactions
• Untreated Addison's disease
• Severe tissue trauma
• Concurrent use of potassium-sparing diuretics, angiotensin-converting enzyme (ACE) inhibitors, or salt substitutes containing potassium
Precautions
Use cautiously in:
• cardiac disease, renal impairment, diabetes mellitus, hypomagnesemia
• pregnant or breastfeeding patients
• children (safety and efficacy not established).
Administration
• Make sure patient is well hydrated and urinating before starting therapy.
☞ Give only as additive to I.V. infusion. Never give by I.V. push or I.M. route, and never give undiluted. Use peripheral line with maximum rate of 40 mEq/hour (with cardiac monitoring).
☞ To ensure that potassium is well mixed in compatible solution, don't add potassium to I.V. bottle in hanging position.
☞ Dilute in compatible I.V. solution. Administer slowly to reduce risk of fatal hyperkalemia.
• Know that maximum infusion rate without cardiac monitoring is 20 mEq/hour. Infusion rates above 20 mEq/hour necessitate cardiac monitoring.
• If patient complains of burning with I.V. administration, decrease flow rate.
• Be aware that potassium preparations are not interchangeable.
• Know that dosages are expressed in mEq of potassium and that potassium acetate contains 10.2 mEq/g.

Adverse reactions
CNS: confusion, unusual fatigue, restlessness, asthenia, flaccid paralysis, paresthesia, absent reflexes
CV: ECG changes, hypotension, arrhythmias, heart block, cardiac arrest
GI: nausea, vomiting, diarrhea, abdominal discomfort, flatulence
Metabolic: hyperkalemia
Musculoskeletal: weakness and heaviness of legs
Respiratory: respiratory paralysis Other: irritation at I.V. site
Interactions
Drug-drug. ACE inhibitors, potassium-sparing diuretics, other potassium-containing preparations: increased risk of hyperkalemia
Drug-diagnostic tests. Potassium: increased level
Drug-food. Salt substitutes containing potassium: increased risk of hyperkalemia
Drug-herbs. Dandelion: increased risk of hyperkalemia
Licorice: decreased response to potassium
Patient monitoring
• Monitor renal function, fluid intake and output, and potassium, creatinine, and blood urea nitrogen levels.
☞ Know that potassium is contra-indicated in severe renal impairment and must be used with extreme caution (if at all) in patients with any degree of renal impairment, because of risk of life-threatening hyperkalemia.
• Assess vital signs and ECG. Watch for arrhythmias.
• Evaluate patient's neurologic status. Stay alert for neurologic complications.
• Monitor I.V. site for irritation.
Patient teaching
• Instruct patient to report unusual pain, redness, swelling, or other reactions at infusion site.
• Advise patient to report nausea, vomiting, confusion, numbness and tingling, unusual tiredness or weakness, or heavy feeling in legs.
• Instruct patient to avoid salt substitutes.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, foods, and herbs mentioned above.