West Nile Virus
Definition
West Nile virus is a mosquito-borne viral illness that can manifest with varying seriousness, ranging from no symptoms or mild flu-like symptoms to brain damage and
death.
Description
West Nile virus (WNV) is a mosquito-borne flavivirus belonging to the
Japanese encephalitis serocomplex, which includes St. Louis
encephalitis, Murray Valley encephalitis, and Kunjin virus. Zoonotically maintained, infections occur generally between late summer and early fall in temperate areas, and throughout the year in southern climates. Although typical manifestation of WNV is asymptomatical, the virus can cross the blood-brain barrier and cause severe illness,
paralysis, and even death in humans and animals.
The WNV was originally isolated in a feverish woman living in the West Nile District of Uganda during 1937. The virus was ecologically characterized in Egypt during the 1950s and later linked to severe human meningoencephalitis in elderly patients during a 1957 outbreak in Israel. Since 1937, subsequent outbreaks of WNV have been reported in Africa, Asia, Australia, Oceania, Western Europe, and the Middle East.
In the summer of 1999, the first North American cases of WNV occurred in the New York City area. It is still unknown how the WNV reached the continental United States, but it is suspected that the transport of infected birds or the international travel of infected humans may have been to blame. After its arrival in the New Work area,, the virus spread rapidly across the United States, as well as northward into Canada and southward into Mexico. In 2002, a severe outbreak of WNV in the United States killed 284 people and caused 2,944 cases of severe brain damage. During another outbreak a year, 9,858 cases of WNV infection were reported, of which over 2,900 were severe in nature and 262 of which resulted in fatality. As of January 2005, the CDC ArboNET has recorded avian or animal WNV infections in every state except Alaska, Hawaii, and Washington. Additionally, the CDC ArboNET recorded human WNV infections in every state except Alaska, Delaware, Hawaii, Massachusetts, New Hampshire, Rhode Island, Vermont, Washington, and West Virginia. Experts believe that WNV is now firmly established in the Western Hemisphere.
Life cycle and transmission
Like most flaviviruses, the WNV is maintained in a natural host-vector-host cycle, where the primary vector is the mosquito. The zoonotic cycle begins with a reservoir host, which is most commonly of avian origin. When a mosquito feeds on the infected bird, the virus is passed to the insect along with the blood meal. The virus then multiplies rapidly within the mosquito's body and salivary glands over the next few days. When the insect feeds on another animal or human, the virus can be transmitted through the bite and cause serious illness.
Most mosquitoes can become infected with the WNV. However, female mosquitoes of the Culex pipiens species are of particular concern, as they live in suburban and urban areas, can survive through the winter, prefer to feed on birds, and frequently bite humans. The Culex pipiens, also known as the house mosquito, is also the most common vector for WNV transmission. Culex restuan, Culex quinquefasciatus, Aedes Albopictus, and Aedes Vexans are also common carriers of the WNV.
Common food sources for mosquitoes, birds represent the primary WNV reservoir species. American crows, in particular, are extremely susceptible to WNV, and have become the virus' primary host population. Indeed, an unusual crow die-off can be used as an excellent indicator for the regional presence of the WNV. The virus has also been identified in more than 250 bird species in the United States, including blue jays, ravens, magpies, sparrows, and starlings. Many in the scientific community believe that the rapid spread of WNV in North America may be due in part to the migratory nature of birds. Infected birds carry the virus with them as they travel in summer and winter, thus acting as reservoirs in their new nesting sites.
Most vertebrates, such as alligators, bats, chipmunks, skunks, squirrels, and rabbits, can also be infected with WNV. Horses, in particular, are commonly infected with WNV. Like humans, the majority of horses suffer either no or mild symptoms, but severe illness and death can and does occur. There are relatively few cases of dogs and cats becoming infected with WNV. Animals of all species exhibiting
fever, weakness, poor coordination, spasms, seizures, and/or personality changes may be infected with WNV.
There is no evidence of WNV transmission from person-to-person through touch, kissing, or other contact. However, there is evidence of WNV transplacental (mother-to-child) transmission, as well as viral transmission through breastfeeding. As such, pregnant mothers should be aware of the presence of WNV in their area and take appropriate precautions. The transmission of WNV has also been evidenced in blood-transfusions and organ transplants; although the current blood supply is now tested for the presence of the WNV. People that are immunocompromised (from disease or
chemotherapy, for example) and people aged 50 and older represent the highest risk group for serious WNV infection.
Causes and symptoms
The exact mechanism of WNV-caused illnesses remains unclear. However, it is suspected that the virus enters the host's blood stream and multiples. It can then develop to the point where it crosses the blood brain-barrier, which separates the blood from the central nervous system. When this occurs, the virus can infect the brain, spinal cord, and other vital systems, creating a potentially deadly inflammatory response.
Symptoms
The incubation period for WNV after infection typical ranges between 3 to 14 days. Eighty percent of infected persons will exhibit no clinically apparent symptoms whatsoever. Roughly 20% of infected persons will exhibit a series of mild flu-like symptoms, also known as West Nile Fever. These mild symptoms can persist for 3 to 6 days, possibly weeks, and include:
- eye pain
- fever
- headache
- loss of appetite
- lymphadenopathy (abnormal enlargement of the lymph nodes)
- malaise (nonspecific bodily discomfort)
- myalgia (nonspecific muscular pain/tenderness)
- nausea
- rash (on the neck, torso, and limbs)
- vomiting
In rare cases, approximately 1 in 150 cases (0.7%), WNV can cross the blood-brain barrier and develop into a severe neuroinvasive disease. Immunocompromised and elderly (>50 years of age) patients are at an increased risk for developing more severe syndromes; a 20-fold increase in incidence among older patients. Symptoms indicating the possible presence of severe West Nile-related syndromes include:
- severe headache
- high fever
- acute muscle weakness
- neck stiffness
- convulsions and tremors
- disorientation and stupor
- paralysis
- coma
People exposed to WNV infection, especially the immunocompromised and elderly, should contact their health provider immediately if they develop a severe headache accompanied by high fever.
Typically, severe WNV syndromes manifest as one of three syndromes: West Nile encephalitis (inflammation of the brain); West Nile
meningitis (inflammation of the meninges of the brain and spinal cord); or West Nile meningoencephalitis (inflammation of both the brain and the meninges). These three syndromes can cause severe brain damage and even death. Severe WNV disease carries a mortality rate ranging between 3% and 15%, with elderly patients suffering the highest mortality rate. The majority of these deaths are as a result of complication attributable to West Nile meningoencephalitis. Additionally, severe WNV disease can cause acute vision loss due to inflammatory disorders of the eye, such as chorioretinitis,
optic neuritis, retinal
vasculitis, uveitis, and vitritis. Less frequently, the patient can exhibit acute flaccid paralysis, similar to poliomyelitis (
polio) or Guillain-Barré syndrome, caused by inflammation of the spinal cord and/or damage to the peripheral nerves. In some severe cases, this acute flaccid paralysis can disrupt muscles that control breathing and result in
respiratory failure.
Diagnosis
A proper diagnosis of WNV infection depends heavily upon clinical presentation, laboratory testing, and patient history. Patients with a known susceptibility to WNV (the elderly and immunocompromised) that exhibit symptoms during the late spring to early fall, or at any time in warmer climates, should be tested for WNV and other arboviral infections. Additionally, health providers should remain constantly aware of the local presence of WNV activity, such as reports of recent animal and/or human cases. Similarities of symptomology between and serological cross-reactivity of WNV and other flaviviruses, may lead to confusion and an incorrect diagnosis. Health providers must use thorough laboratory testing to differentiate WNV antibodies from those of other arboviruses.
Symptomatic WNV infection can be classified as either non-neuroinvasive or neuroinvasive, with each being identified according to certain criteria.
Non-neuroinvasive
The majority of WNV infections are asymptomatic. In approximately 20% of WNV cases, clinically recognizable symptoms can manifest. However, to be clinically classified as non-neuroinvasive West Nile disease, the following must be true:
- no neuroinvasive symptomology
- presence of fever without other recognizable cause
- four-fold or greater increase in serum antibody titer
- virus isolated from and or deomonstrated in blood, tissue, cerebrospinal fluid (CSF), or other bodily fluid
- virus-specific immunoglobulin M (IgM antibodies demonstrated in CSF through antibody-capture methods
Neuroinvasive
In the rare cases (0.7%) of West Nile disease, the virus crosses the blood-brain barrier and manifests in severe and life-threatening symptomology. Clinical confirmation of neuroinvasive of neuroinvasive disease requires the presence of a fever and at least one of the following:
- acutely altered mental status, such as disorientation, stupor, or coma
- acute central or peripheral neurological difficulties, such as paralysis, nerve palsy, sensory deficits, and abnormal muscle function
- an increased white blood cell concentration in the CSF coupled with symptoms of meningitis, such as severe headache and neck pain
Treatment
Currently, there are no treatment modalities for WNV infection. Instead, supportive care is utilized to treat the varying symptoms and syndromes associated with the various West Nile diseases. Although milder symptoms can be treated at home, severe symptoms can require hospitalization. Treatment of severe symptoms may require the use of intravenous infusions, airway and respiratory management and support, and use of preventative measures against secondary infection.
Prognosis
The majority of WNV infections will manifest asymptomatically. West Nile fever offers an excellent prognosis associated with quick recovery and no adverse side-effects. The majority of symptoms will resolve within a few days or weeks of manifestation.
However, the prognosis is not a positive for patients suffering the more severe syndromes attributable to WNV infection. Symptoms of West Nile encephalitis, West Nile meningitis, and West Nile meningoencephalitis can last for several weeks, as well as cause severe and permanent neurological damage. Inflammation can interfere with the brain and central nervous system and result in death, especially amongst the elderly population. Patients with West Nile poliomyelitis may suffer prolonged muscle weakness and loss of motor control. Long-term
rehabilitation is typically required and a full recovery is not assured. If the poliomyelitis affects muscles used for breathing, death from respiratory failure may result.
Prevention
Although there is a vaccine used for horses and exotic birds in zoos, there is no WNV vaccine for humans at the current time. Several pharmaceutical companies, however, have WNV vaccines in development.
Prevention techniques of WNV typically coincide with avoidance measures against mosquito bites; the primary source of the virus. These include the use of insect repellant (with 5% to 20% DEET) on exposed body parts, wearing loose-fitting clothes over the limbs and torso while outdoors, using mosquito coils and/or citronella candles outdoors, and limiting outdoor activities during peak biting periods and/or in areas with high mosquito density. While camping outdoors, knockdown spray or bed netting with pyrethrum is suggested. Mosquito eradication programs have been instituted in most major cities. Public health authorities can utilize United States Environmental Protection Agency-approved "adulticidies" in areas suspected of the presence of WNV.
The Culex pipiens mosquito is the primary vector of WNV transmission and is also commonly live and feed in urban areas. Special precautions should be taken to reduce exposure to these potentially infected insects. Screen doors and enclosed porches can help keep mosquitoes from coming into the house. It should be noted that studies have shown that mosquito control devices such as "bug zappers" and CO2-baited traps do not significantly reduce the risk of being bitten.
Removing potential mosquito breeding areas from near the home and from the neighbourhood can further reduce the risk of bites. Any container which can collect half an inch of standing water can become a potential breeding site in as little as five days. Old tires, empty plant pots, and empty trashcans should be removed, while water sources like ponds or birdbaths should be cleaned regularly. Standing water on any property should be drained, such as from clogged eves. Swimming pools and hot tubs should be properly covered and chlorinated to prevent mosquitoes breeding in them.
Books
Mackenzie J., A.D.T. Barrett, and V. Deubel (eds.) Japanese encephalitis and West Nile viruses Berlin: Springer, 2002.
Periodicals
Fryer, J. "Interview with Joe Garret" Texas Journal of Rural Health 20 (#3, 2001): 5-7.
O'Leary D., A. Marfin, S. Montgomery, A. Kipp, J. Lehman, B. Biggerstaff, et al. "The Epidemic of West Nile Virus in the United States, 2002." Vector-borne and Zoonotic Diseases 4(March 2002): 61-.
Peterson L, and J. Roehrig. "West Nile Virus: A Reemerging Gobal Pathogen." Emerging Infectious Diseases 7(July-August 2001): 611-614.
Rappole J., S. Derrickson, and Z. Hubalek. "Migratory Birds and Spread of West Nile Virus in the Western Hemisphere." Emerging Infectious Diseases 6 (July-August 2000): 319-328.
Huhn G', J. Sejvar, S. Montgomery, and M. Dworkin. "West Nile Virus in the United States: An Update on an Emerging Infectious Disease." American Family Physician 68 (August 2003): 653-672.
Organizations
Centers for Disease Control and Prevention. 1600 Clifton Rd, Atlanta, GA 30333. (800)311-3435. http://www.cdc.gov.
Key terms
Flavivirus — An arbovirus that can cause potentially serious diseases, such as dengue, yellow fever, Japanese encaphilitis, and West Nile fever.
Guillain-Barré — A disorder in which the body's immune system attacks part of the peripheral nervous system. Weakness, tingling, and abnormal sensations in the arms and upper body can progress until the muscles become totally disabled and the patient is effectively paralyzed.
Meninges — A series of membranous layers of connective tissue that protect the central nervous system (brain and spinal cord). Damage or infection to the meninges, such as in meningitis, can cause serious neurological damage and even death.
Zoonotic diseases — Diseases caused by infectious agents that can be transmitted between (or are shared by) animals and humans. This can include transmission through the bite of an insect, such as a mosquito.
Gale Encyclopedia of Medicine. Copyright 2008 The Gale Group, Inc. All rights reserved.
virus
[vi´rus] any member of a unique class of infectious agents, which were originally distinguished by their smallness (hence, they were described as “filtrable” because of their ability to pass through fine ceramic filters that blocked all cells, including bacteria) and their inability to replicate outside of and without assistance of a living host cell. Because these properties are shared by certain bacteria (
rickettsiae, chlamydiae), viruses are now characterized by their simple organization and their unique mode of replication. A virus consists of genetic material, which may be either DNA or RNA, and is surrounded by a protein coat and, in some viruses, by a membranous envelope.
Unlike cellular organisms, viruses do not contain all the biochemical mechanisms for their own replication; they replicate by using the biochemical mechanisms of a host cell to synthesize and assemble their separate components. (Some do contain or produce essential enzymes when there is no cellular enzyme that will serve.) When a complete virus particle (
virion) comes in contact with a host cell, only the viral nucleic acid and, in some viruses, a few enzymes are injected into the host cell.
Within the host cell the genetic material of a DNA virus is replicated and transcribed into messenger RNA by host cell enzymes, and proteins coded for by viral genes are synthesized by host cell ribosomes. These are the proteins that form the
capsid (protein coat); there may also be a few enzymes or regulatory proteins involved in assembling the capsid around newly synthesized viral nucleic acid, in controlling the biochemical mechanisms of the host cell, and in lysing the host cell when new virions have been assembled. Some of these may already have been present within the initial virus, and others may be coded for by the viral genome for production within the host cell.
Because host cells do not have the ability to replicate “viral RNA” but are able to transcribe messenger RNA, RNA viruses must contain enzymes to produce genetic material for new virions. For certain viruses the RNA is replicated by a viral enzyme (
transcriptase) contained in the virion, or produced by the host cell using the viral RNA as a messenger. In other viruses a
reverse transcriptase contained in the virion transcribes the genetic message on the viral RNA into DNA, which is then replicated by the host cell.
Reverse transcriptase is actually a combination of two enzymes: a
polymerase that assembles the new DNA copy and an
RNase that degrades the source RNA.
In viruses that have membranes, membrane-bound viral proteins are synthesized by the host cell and move, like host cell membrane proteins, to the cell surface. When these proteins assemble to form the capsid, part of the host cell membrane is pinched off to form the envelope of the virion.
Some viruses have only a few genes coding for capsid proteins. Other more complex ones may have a few hundred genes. But no virus has the thousands of genes required by even the simplest cells. Although in general viruses “steal” their lipid envelope from the host cell, virtually all of them produce “envelope proteins” that penetrate the envelope and serve as receptors. Some envelope proteins facilitate viral entry into the cell, and others have directly pathogenic effects.
Some viruses do not produce rapid lysis of host cells, but rather remain latent for long periods in the host before the appearance of clinical symptoms. This carrier state can take any of several different forms. The term
latency is used to denote the interval from infection to clinical manifestations. In the
lentiviruses, it was formerly mistakenly believed that virus was inactive during this period. The true situation is that
lentiviruses are rapidly replicating and spawning dozens of quasi-species until a particularly effective one overruns the ability of the host's
immune system to defeat it. Other viruses, however, such as the
herpesviruses, actually enter a time known as “viral latency,” when little or no replication is taking place until further replication is initiated by a specific trigger. For many years all forms of latency were thought to be identical, but now it has been discovered that there are different types with basic and important distinctions.
In viral latency, most of the host cells may be protected from infection by immune mechanisms involving antibodies to the viral particles or
interferon. Cell-mediated immunity is essential, especially in dealing with infected host cells. Cytotoxic
lymphocytes may also act as antigen-presenting
cells to better coordinate the
immune response. Containment of virus in mucosal tissues is far more complex, involving follicular dendritic cells and Langerhans
cells.
Some enveloped RNA viruses can be produced in infected cells that continue growing and dividing without being killed. This probably involves some sort of intracellular regulation of viral growth. It is also possible for the DNA of some viruses to be incorporated into the host cell DNA, producing a carrier state. These are almost always
retroviruses, which are called
proviruses before and after integration of viral DNA into the host genome.
Few viruses produce toxins, although viral infections of bacteria can cause previously innocuous bacteria to become much more pathogenic and toxic. Other viral proteins, such as some of the
human immunodeficiency virus, appear to be actively toxic, but those are the exception, not the rule.
However, viruses are highly antigenic. Mechanisms of pathologic injury to cells include cell
lysis; induction of cell
proliferation (as in certain
warts and
molluscum contagiosum); formation of giant cells, syncytia, or intracellular inclusion bodies caused by the virus; and perhaps most importantly, symptoms caused by the host's
immune response, such as inflammation or the deposition of antigen-antibody complexes in tissues.
Because viral reproduction is almost completely carried out by host cell mechanisms, there are few points in the process where stopping viral reproduction will not also kill host cells. For this reason there are no chemotherapeutic agents for most viral diseases.
acyclovir is an antiviral that requires viral proteins to become active. Some viral infections can be prevented by vaccination (
active immunization), and others can be treated by
passive immunization with
immune globulin, although this has been shown to be effective against only a few dozen viruses.
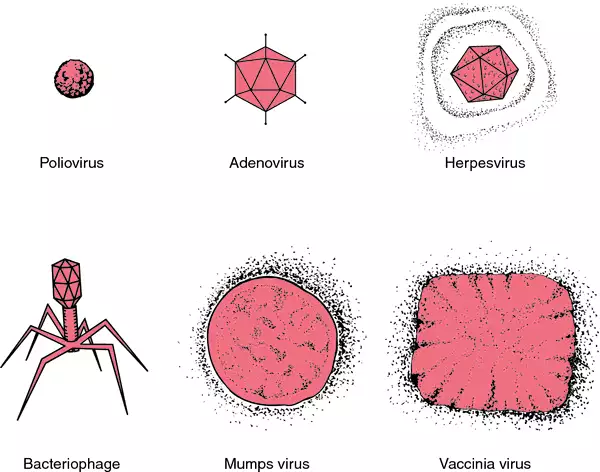
Comparison of shapes and sizes of viruses.
attenuated virus one whose pathogenicity has been reduced by serial animal passage or other means.
bacterial virus one that is capable of producing transmissible lysis of bacteria; see also
bacteriophage.
defective virus one that cannot be completely replicated or cannot form a protein coat; in some cases replication can proceed if missing gene functions are supplied by other viruses; see also
helper virus.
dengue virus a flavivirus, existing as four antigenically related but distinct types (designated 1, 2, 3, and 4), that causes both the classic and hemorrhagic forms of
dengue.
DNA virus a virus whose
genome consists of DNA.
Ebola virus an RNA virus almost identical to the
Marburg virus but serologically distinct; it causes a disease similar to that caused by the Marburg virus.
encephalomyocarditis virus an enterovirus that causes mild aseptic meningitis and encephalomyocarditis.
enteric orphan v's orphan viruses isolated from the intestinal tract of humans and other animals.
Epstein-Barr virus (EBV) a herpeslike virus that causes infectious mononucleosis and is associated with Burkitt's lymphoma and nasopharyngeal carcinoma; see also
epstein-barr virus.
fixed virus a virus whose virulence and incubation period have been stabilized by serial passage and have remained fixed during further transmission, as opposed to a
street virus.
helper virus one that aids in the development of a defective virus by supplying or restoring the activity of the viral gene or enabling it to form a protein coat.
hepatitis A virus (HAV) any virus of the genus
Hepatovirus that causes
hepatitis a. This has the most rapid onset of the hepatitis viruses affecting humans; transmission is easier than for the
hepatitis B and
C viruses, but infection generally does not persist. While infection with this virus alone is usually not life-threatening, coincident infection with
hepatitis C virus is generally rapidly fatal.
hepatitis B virus (HBV) a species of genus
Orthohepadnavirus that causes
hepatitis b.
hepatitis C virus a species of genus
Hepacivirus that causes
hepatitis c; its latency period may last 30 years or more.
hepatitis D virus (HDV) (
hepatitis delta virus) an unclassified defective RNA virus, thought of as a parasite of the
hepatitis B virus and transmitted in the same manner; it requires enzymes and other assistance from HBV to replicate. This virus magnifies the pathogenicity of
hepatitis B virus many times and is the etiologic agent of
hepatitis d.
hepatitis E virus an enterically transmitted calicivirus that causes hepatitis e.
hepatitis G virus (HGV) a parenterally transmitted flavivirus originally isolated from a patient with chronic hepatitis; most infections are benign, and it is uncertain what role, if any, HGV plays in the etiology of liver disease.
hepatotropic virus a virus that primarily affects the liver, such as the
hepatitis viruses.
herpes virus herpesvirus.
herpes simplex virus former name for any virus that causes
herpes simplex, now called human herpesviruses; see
herpesvirus.
human immunodeficiency virus (HIV) either of two species of
lentiviruses that cause
acquired immunodeficiency syndrome (AIDS). HIV-1 is found around the world and HIV-2 is found primarily in West Africa. Progression of HIV-2 infection to AIDS is generally slower and less extreme than that of HIV-1. The virus is believed to induce permanent infection and has a propensity toward a subset of
T lymphocytes called the CD4
cells. The infected cells become dysfunctional and eventually the host's
immune system is overwhelmed or exhausted; death ensues, usually as a result of infection. The virus is not transmitted through casual contact; the most common routes of transmission are through sexual intercourse, direct exposure to contaminated blood, and transplacental transmission from mother to fetus.
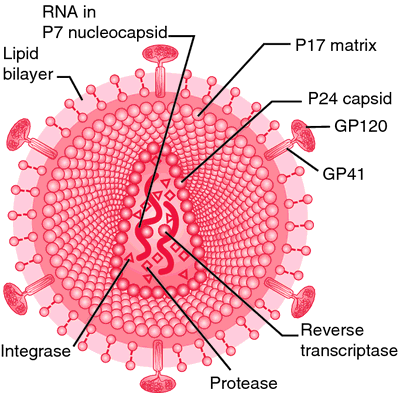
Human immunodeficiency virus: retrovirus particle. From Copstead, 1995.
human T-lymphotropic virus (HTLV) either of two related species of
retroviruses that have an affinity for the helper
cell type of T lymphocytes. HTLV-1 causes chronic infection and is associated with
adult T-cell leukemia and a type of
myelopathy. HTLV-2 has been isolated from an atypical variant of
hairy cell leukemia and from patients with other hematological disorders, but no clear association with disease has been established.
influenza virus any of a group of
orthomyxoviruses that cause
influenza; there are at least three serotypes or species (A, B, and C). Serotype A viruses are subject to major antigenic changes (
antigenic shifts) as well as minor gradual antigenic changes (
antigenic drift) and cause widespread epidemics and pandemics. Serotypes B and C are chiefly associated with sporadic epidemics.
La Crosse virus a virus of the California serogroup of the genus Bunyavirus, the etiologic agent of La Crosse encephalitis.
latent virus one that ordinarily occurs in a noninfective state and is demonstrable by indirect methods that activate it.
lytic virus one that is replicated in the host cell and causes death and lysis of the cell.
maedi/visna virus a
lentivirus that is the etiologic agent of a type of
pneumonia in sheep.
Marburg virus an RNA virus occurring in Africa, transmitted by insect bites, and causing
marburg virus disease.
mumps virus a
paramyxovirus that causes
mumps and sometimes tenderness and swelling of the testes, pancreas, ovaries, or other organs.
Norwalk virus a
calicivirus that is common cause of epidemics of acute
gastroenteritis, with diarrhea and vomiting that last 24 to 48 hours.
oncogenic v's an epidemiologic class of viruses that are acquired by close contact or injection and cause usually persistent infection; they may induce cell transformation and malignancy.
orphan v's viruses isolated in tissue culture, but not found specifically associated with any disease.
parainfluenza virus any of various
paramyxoviruses that cause upper respiratory tract disease of varying severity.
rabies virus an RNA virus of the rhabdovirus group that causes
rabies.
respiratory syncytial virus (RSV) any of a genus of single-stranded
paramyxoviruses; the name is derived from the type of disease produced (respiratory infection) and the microscopic appearance of the viruses in cell cultures. RSV can cause a wide variety of respiratory disorders ranging from a mild cold to serious or even fatal disease of the lung in the very young and very old. It regularly produces an outbreak of infection each winter and virtually disappears in the summer months. The most severe infections in children are in the very young, especially those who are preterm, immunologically compromised, or suffering from a congenital heart defect or preexisting lung disorder. Adults at risk for infection include parents and others who are repeatedly exposed to young children, for example, pediatric nurses and day care attendants. The course of infection tends to be milder in adults than in children and about 15 per cent of affected adults have no symptoms. In the very elderly these infections may have the same degree of seriousness and clinical manifestations as in the very young.
RNA virus a virus whose
genome consists of RNA.
satellite virus a strain of virus unable to replicate except in the presence of
helper virus; considered to be deficient in coding for
capsid formation.
simian immunodeficiency virus (SIV) a
lentivirus closely related to
human immunodeficiency virus that causes inapparent infection in African green monkeys and a disease resembling
acquired immunodeficiency syndrome in macaques and chimpanzees.
slow virus any virus that remains latent for long periods in the infected host before the appearance of clinical symptoms.
street virus virus from a naturally infected animal, as opposed to a laboratory-adapted strain of the virus.
vaccinia virus a species of
orthopoxvirus that does not occur in nature and has been propagated for many years only in the laboratory for use as an active vaccine against
smallpox. The present virus is derived from the original one used by Jenner, obtained from the lesions of
cowpox, but the origin of the original virus remains unclear.
varicella-zoster virus former name for human herpesvirus 3; see herpesvirus.
variola virus the virtually extinct
orthopoxvirus that is the etiologic agent of
smallpox. No natural infection has occurred since 1977, and no reservoir of the virus now exists.
West Nile virus a virus of the genus
Flavivirus, the cause of West Nile
encephalitis; it is transmitted by
Culex mosquitoes, with wild birds serving as the reservoir. It was originally endemic in Africa, Asia, and Europe, but recently spread to North America.
Miller-Keane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health, Seventh Edition. © 2003 by Saunders, an imprint of Elsevier, Inc. All rights reserved.

