cefazolin sodium
Pharmacologic class: First-generation cephalosporin
Therapeutic class: Anti-infective
Pregnancy risk category B
Action
Interferes with bacterial cell-wall synthesis, causing cell to rupture and die
Availability
Powder for injection: 500 mg, 1 g, 10 g, 20 g
Premixed containers: 500 mg/50 ml in dextrose 5% in water (D5W), 1 g/50 ml in D5W
Indications and dosages
➣ Respiratory tract infections caused by group A beta-hemolytic streptococci, Klebsiella species, Haemophilus influenzae, and Staphylococcus aureus; skin infections caused by S. aureus and beta-hemolytic streptococci; biliary tract infections caused by Escherichia coli, Klebsiella species, Proteus mirabilis, and S. aureus; bone and joint infections caused by S. aureus; genital infections caused by E. coli, Klebsiella species, P. mirabilis, and strains of enterococci; septicemia caused by E. coli, Klebsiella species, P. mirabilis, S. aureus, and S. pneumoniae; endocarditis caused by S. aureus or beta-hemolytic streptococci
Adults: For mild infections, 250 to 500 mg q 8 hours I.V. or I.M. For moderate to severe infections, 500 to 1,000 mg I.V. or I.M. q 6 to 8 hours. For life-threatening infections, 1,000 to 1,500 mg I.M. or I.V. q 6 hours, to a maximum dosage of 6 g/day.
Children: For mild to moderate infections, 25 to 50 mg/kg/day I.V. or I.M. in divided doses t.i.d. or q.i.d. For severe infections, 100 mg/kg/day I.V. or I.M. in divided doses t.i.d. or q.i.d.
➣ Acute uncomplicated urinary tract infections (UTIs) caused by E. coli, Klebsiella species, P. mirabilis, and strains of Enterococcus and Enterobacter species
Adults: 1 g I.V. or I.M. q 12 hours
➣ Surgical prophylaxis
Adults: 1g I.V. or I.M. 30 to 60 minutes before surgery, then 0.5 to 1 g I.V. or I.M. q 6 to 8 hours for 24 hours. If surgery exceeds 2 hours, another 0.5- to 1-g dose I.M. or I.V. may be given intraoperatively.
➣ Pneumococcal pneumonia
Adults: 500 mg I.M. or I.V. infusion q 12 hours
Dosage adjustment
• Renal impairment
• Elderly patients
Contraindications
• Hypersensitivity to cephalosporins or penicillins
Precautions
Use cautiously in:
• renal impairment, phenylketonuria
• history of GI disease (especially colitis)
• emaciated patients
• elderly patients
• pregnant or breastfeeding patients
• children.
Administration
• Obtain specimens for culture and sensitivity testing as needed before starting therapy.
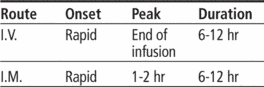
• For intermittent I.V. infusion, administer in volume-control set or in separate, secondary I.V. container over 30 to 60 minutes.
• For direct I.V. injection, dilute reconstituted dose in 5 ml of sterile water for injection and administer slowly over 3 to 5 minutes.
• Don't mix premixed solution with other drugs.
☞ Don't use flexible container in series connections because of risk of air embolism.
• For I.M. use, reconstitute with sterile water for injection, bacteriostatic water, or normal saline solution for injection. Shake well until dissolved.
• Inject I.M. into large muscle mass.
Adverse reactions
CNS: headache, lethargy, confusion, hemiparesis, paresthesia, syncope, seizures
CV: hypotension, palpitations, chest pain, vasodilation
EENT: hearing loss
GI: nausea, vomiting, diarrhea, abdominal cramps, oral candidiasis, pseudomembranous colitis
GU: vaginal candidiasis, nephrotoxicity
Hematologic: lymphocytosis, eosinophilia, bleeding tendency, hemolytic anemia, hypoprothrombinemia, neutropenia, thrombocytopenia, agranulocytosis, bone marrow depression
Hepatic: hepatic failure, hepatomegaly
Musculoskeletal: arthralgia
Respiratory: dyspnea
Skin: urticaria, maculopapular or erythematous rash
Other: chills, fever, superinfection, anaphylaxis, serum sickness
Interactions
Drug-drug. Aminoglycosides, loop diuretics: increased risk of nephrotoxicity
Anticoagulants: increased anticoagulant effect
Chloramphenicol: antagonistic effect
Probenecid: decreased excretion and increased blood level of cefazolin
Drug-diagnostic tests. Alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, bilirubin, blood urea nitrogen, creatinine, eosinophils, gamma-glutamyltransferase, lactate dehydrogenase: increased levels
Coombs' test, urinary 17-ketosteroids, nonenzyme-based urine glucose tests (such as Clinitest): false-positive results
Hemoglobin, platelets, white blood cells: decreased values
Drug-behaviors. Alcohol use: acute alcohol intolerance (disulfiram-like reaction) if alcohol is consumed within 72 hours of drug administration
Patient monitoring
☞ If patient is receiving high doses, monitor for extreme confusion, tonic-clonic seizures, and mild hemiparesis.
• Monitor CBC, prothrombin time, and kidney and liver function test results.
• Watch for signs and symptoms of superinfection and other serious adverse reactions.
• Be aware that cross-sensitivity to penicillins may occur.
Patient teaching
• Tell patient to report reduced urinary output, persistent diarrhea, bruising, or bleeding.
• Instruct patient to take drug exactly as prescribed and to complete full course of therapy even when he feels better.
• As appropriate, review all other significant and life-threatening adverse reactions and interactions, especially those related to the drugs, tests, and behaviors mentioned above.